Patients received Cetuximab may develop acquired drug resistance. The reason is a mutation in the mitogen-activated protein kinase (MAPK) pathway, the RAS gene is the most important mutation of the pathway. Moreover, mutations of RAS genes in blood are closely related to mutations in tumor tissues. Therefore, detection of RAS mutation with blood may now become an alternative to routine clinical practice. The purpose of this study is to use ctDNA auxiliary detection tools in blood to detect the prevalence of RAS mutation in plasma form the patients treated with cetuximab. We can predict the benefit of tumor response and anti-EGFR drug therapy.
The MassARRAY platform is used to evaluate the mutations of the RAS gene in plasma. Blood sample collection points are included when they meet the admission conditions, once every 3 months during the first-line treatment and within 2 weeks when the disease treatment is ineffective (Figure 1). The primary endpoint of the study is the mutation rate of the RAS gene during patients receiving cetuximab (Cetuximab) as first-line treatment, and to observe the correlation between RAS gene mutations and treatment efficacy and survival.
The RAS gene mutation rate among Chinese people who receive cetuximab (Cetuximab) as the first-line treatment is about 9.3%, and the RAS gene mutation time is 3 months earlier than the patient's imaging confirms that the treatment is ineffective. Moreover, the disease progression-free survival rate and overall survival rate of patients in this group with RAS gene mutations are significantly worse than those without mutations (P values are 0.002 and 0.027 respectively).
This study demonstrates that using the MassARRAY platform to assess the mutation status of the RAS gene in plasma can be an effective method for detecting early tumor treatment response and predicting the effectiveness of anti-EGFR drugs. A concise graphic abstract of this study is shown in Figure 2 .
Graphical Abstract
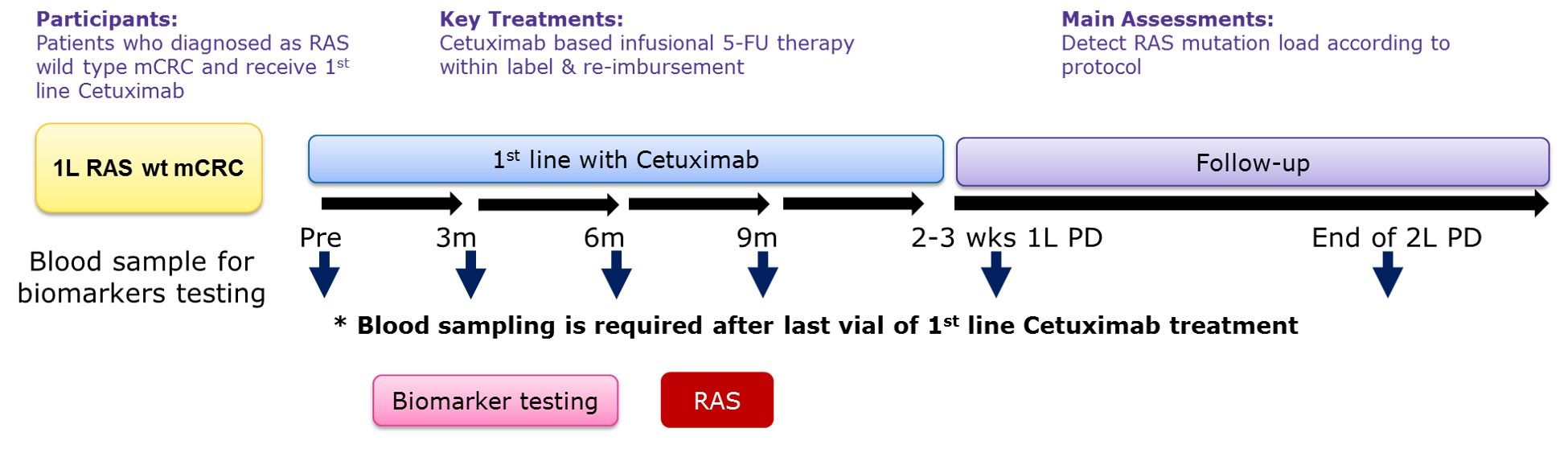
(Figure 1)
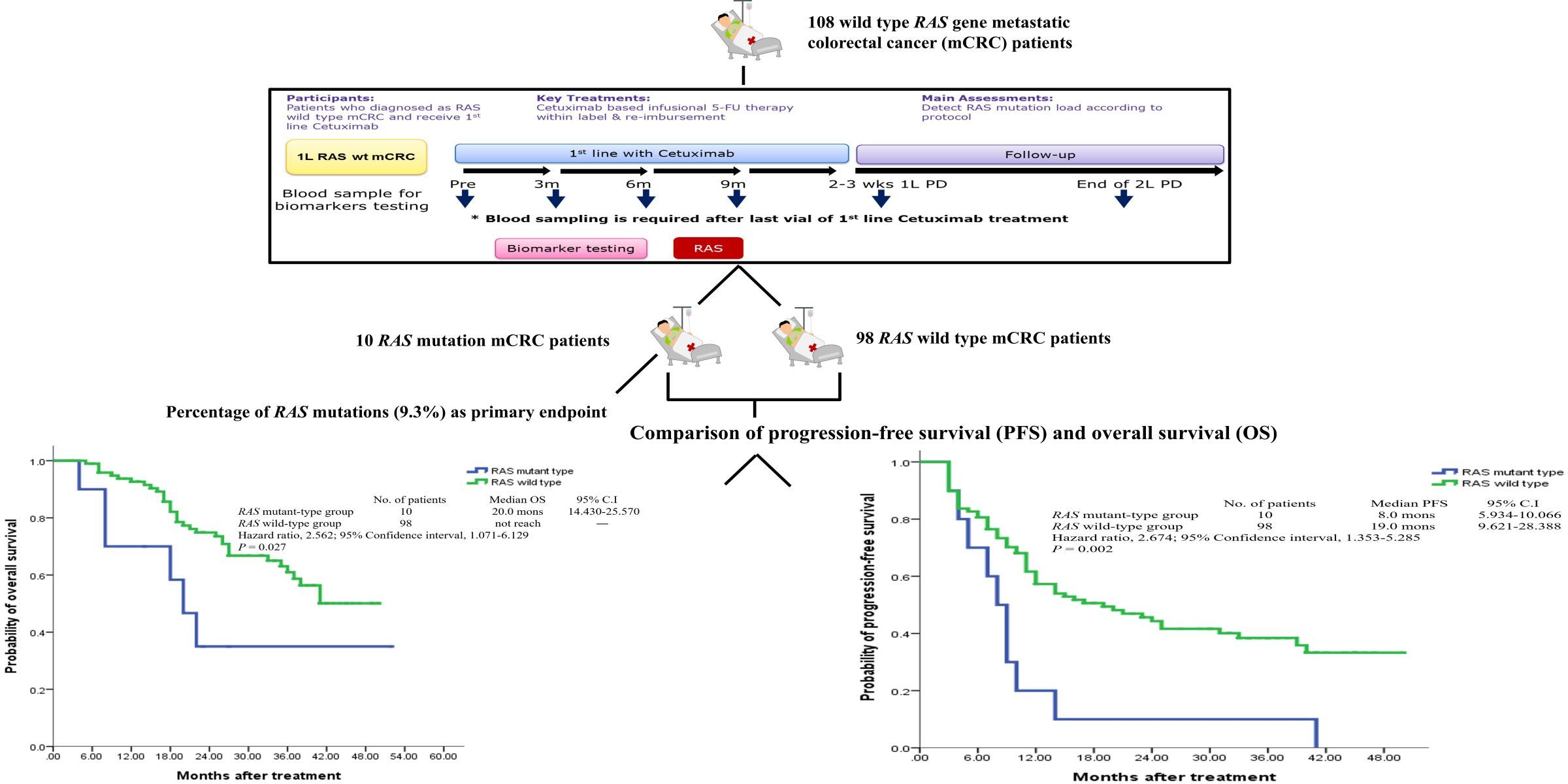
(Figure 2)
Application and Highlights:
1. By simply collect blood regularly, we can find out whether a mutation in the RAS gene. If mutation occur, the medication can be changed early for effective treatment.
2. Compared with the acquisition of invasive tissue specimens, blood testing can make patients more accepting, thereby increasing the testing rate.
3. The MassARRAY platform is cheaper than the NGS method, it’s helpful for clinical promotion.
Research Team Members:
Wang Jaw-Yuan Prof, Chen Li-Tzong Prof., Tsai Hsiang-Lin A.P., Wu Chang-Han phD.
Representative Department:
Center for Cancer Research, Kaohsiung Medical University
Introduction of Research Team:
The research field of Professor Wang Jaw-Yuan is colorectal cancer and its treatment. It focuses on combining basic and clinical medical oncology in translational medicine and clinical potential application value. The key development technologies include cancer gene detection, tumor molecular biology, targeted drugs, Immuno-drugs and minimally invasive precision surgery.
Contact Email:
cy614112@ms14.hinet.net; jawyuanwang@gmail.com
Publication: Br J Cancer. 2023, 129, 947-955.
Full-Text Article:http://doi.org/10.1038/s41416-023-02366-z
